We met Emma as she applied to be one of the METUPUK Darker Side of Pink ladies with our campaign that started in 2021 which focused on metastatic breast cancer,
Kit Dzeryn
Sadly our Kit @Diamondtrees11 died on Monday 7th May. Her husband Phillip contacted us with the tragic news. We didn’t meet Kit but she was an integral part of METUPUK
The Irony of Metastatic Breast Cancer – Mary Huckle

Many of our followers on social media will remember Mary Huckle and her tireless campaigning for #metastaticbreastcancer awareness. This blog post is the last content she wrote for us, a
METUPUK are delighted by the Scottish Medicines Consortium (SMC) acceptance of Enhertu (trastuzumab deruxtecan) for routine use on the NHS.
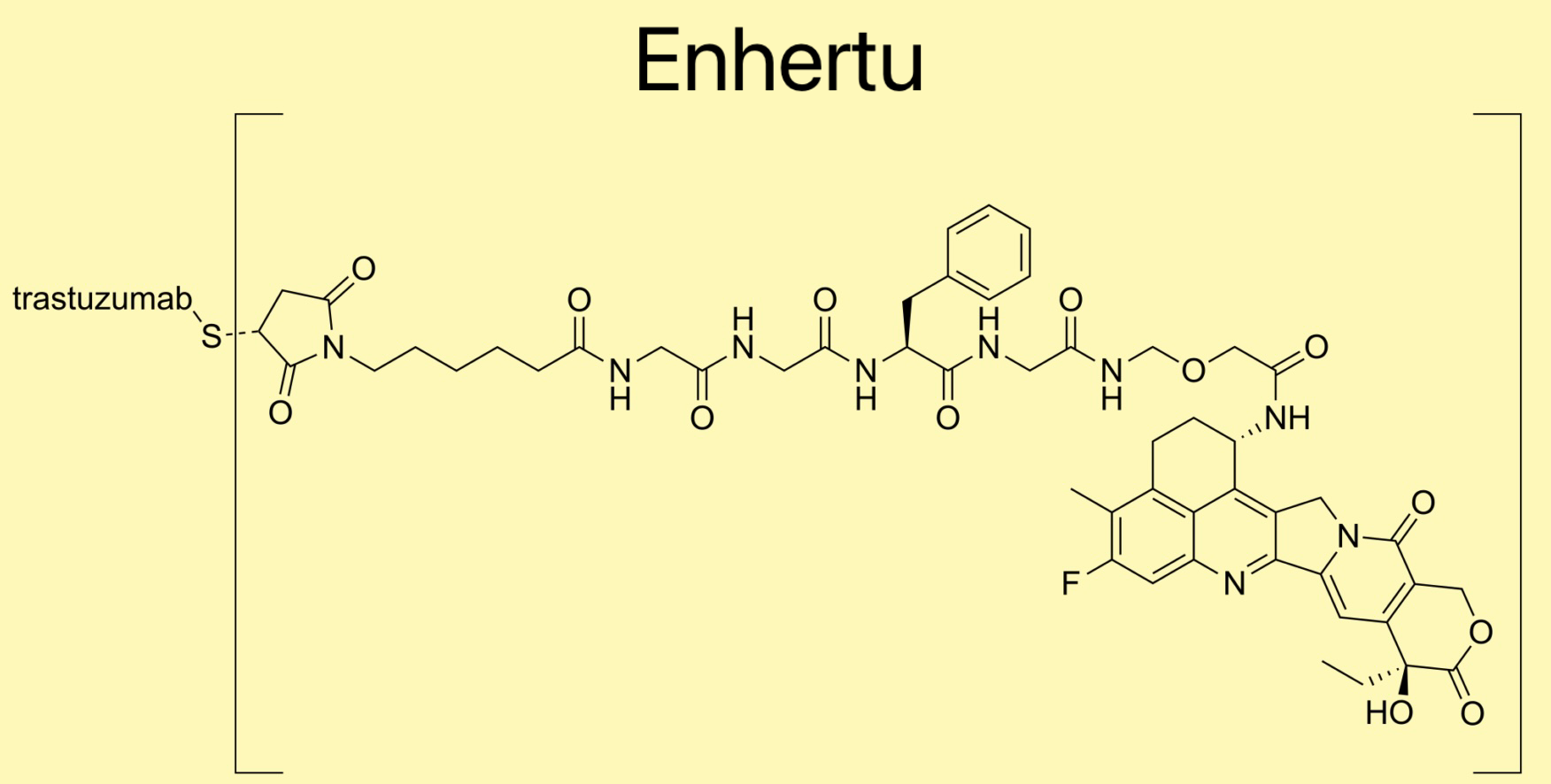
METUPUK are delighted by the Scottish Medicines Consortium (SMC) acceptance of Enhertu (trastuzumab deruxtecan) for routine use on the NHS. The Scottish Medicines Consortium (SMC) has accepted: Trastuzumab deruxtecan (Enhertu)
Piqray (Alpelisib) denied by Scottish Medicines Consortium

The Scottish Medicines Consortium (SMC) have decided against funding the metastatic breast cancer drug alpelisib (Piqray).
Philippa Hetherington Remembered

We had to confirm back on the 5th November the heartbreaking news that the wonderful Philippa Hetherington had died 💔
She was an absolute force of intellectual nature for breast cancer in general, not just Inflammatory Breast Cancer.
Did you know that the Trodelvy campaign in England for TNBC was HER campaign?
Targeted treatment Piqray with Fulvestrant is approved
METUPUK welcomes the approval of Piqray (alpelisib) plus fulvestrant for the treatment of hormone receptor-positive, HER2-negative, PIK3CA- mutated advanced breast cancer.
Piqray is an exciting new cancer medicine because it is the first targeted treatment option for advanced breast cancer that has a PIK3CA mutation.
Trodelvy Now! Latest Update
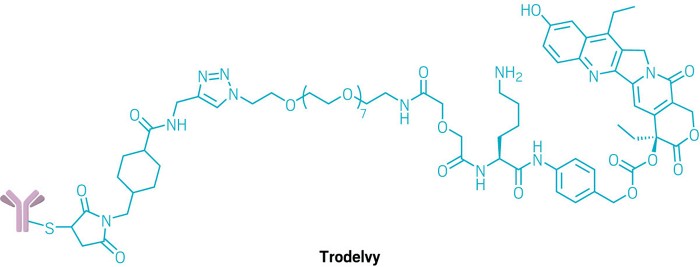
METUPUK are delighted that NICE has accepted Trodelvy (sacituzumab govitecan) for routine use on the NHS in England for the treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer who have received two or more prior lines of systemic therapies. This follows on from the decision to approve Trodelvy in NHS Scotland in March 2022. Wales and Northern Ireland normally follow decisions by NHS England and we hope that Trodelvy will be made available to every patient who is eligible without delay.
Don’t Leave Metastatic Breast Cancer Patients in Wales Behind

A new campaign in Wales, by MetUpUK member Tassia Haines, aims to draw attention to the fact that Metastatic Breast Cancer (MBC) patients feel that they are being failed by the Welsh Government.
Join us as Laura, Mary Helen & Emily discuss primary & secondary breast cancer.
Have you ever wondered what the relationship is like between primary and secondary breast cancer patients when they are talking about cancer?
We’ve put 4 of our MetUpUK members on a zoom call to chat it out – Mary and Laura have secondary cancer and Helen and Emily have had primary breast cancer.
