
Any patient with metastatic breast cancer (MBC) will be familiar with the concept of ‘treatment lines’. Unlike primary breast cancer, where you undergo chemotherapy for a fixed time period, after

Any patient with metastatic breast cancer (MBC) will be familiar with the concept of ‘treatment lines’. Unlike primary breast cancer, where you undergo chemotherapy for a fixed time period, after

METUPUK’ Breast Cancer Awareness Month campaign for October across the UK and on social media channels. Continuing the synergy with The Darker Side of Pink travelling campaign highlighting the 31 women a

A cancer drug which received standing ovation from the Global Medical Community is being withheld from NHS breast cancer patients. METUPUK are devastated by NICE decision not to recommend the drug

METUPUK are saddened by the news that one of our members and friend Asha Umrawsingh has died. Asha’s diagnosis and access to treatment was a roller coaster of how strict

METUPUK (Metastatic Exchange To Unleash Power) attended the 2024 United Kingdom’s Interdisciplinary Breast Cancer Symposium in Birmingham, hosted by Breast Cancer Now and supported by numerous partners. It was a
METUPUK are delighted by the National Institute of Clinical Excellence (NICE) decision to recommend Talazoparib (Talzenna ®, Pfizer Ltd.) for treating HER2-negative, locally advanced or metastatic breast cancer with germline
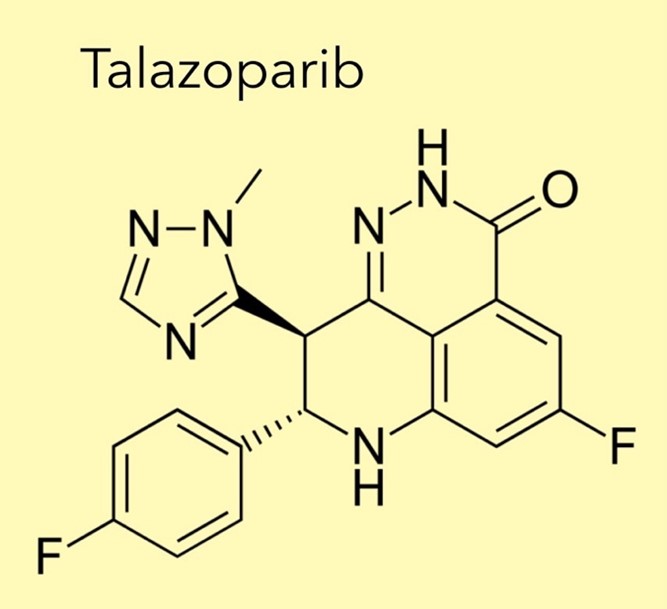
METUPUK are disappointed by the National Institute of Clinical Excellence (NICE) decision to not recommend talazoparib (Talzenna ®, Pfizer Ltd.) for treating HER2-negative, locally advanced or metastatic breast cancer with

METUPUK historically maintained their own in-house clinical trial database for metastatic trials in the UK as there was no one source that contained them all. It used the key sites used across the UK; Cancer Research UK, Be Part of Research, ISRCTN and ClinicalTrials.gov.
Leaping forward to 2022, surely by now one of them must be THE accurate, easily searchable source of breast cancer trials? If not, how do our oncologists quickly and accurately find the best trials for their metastatic patients?
And where do patients start when looking for a metastatic trial themselves?
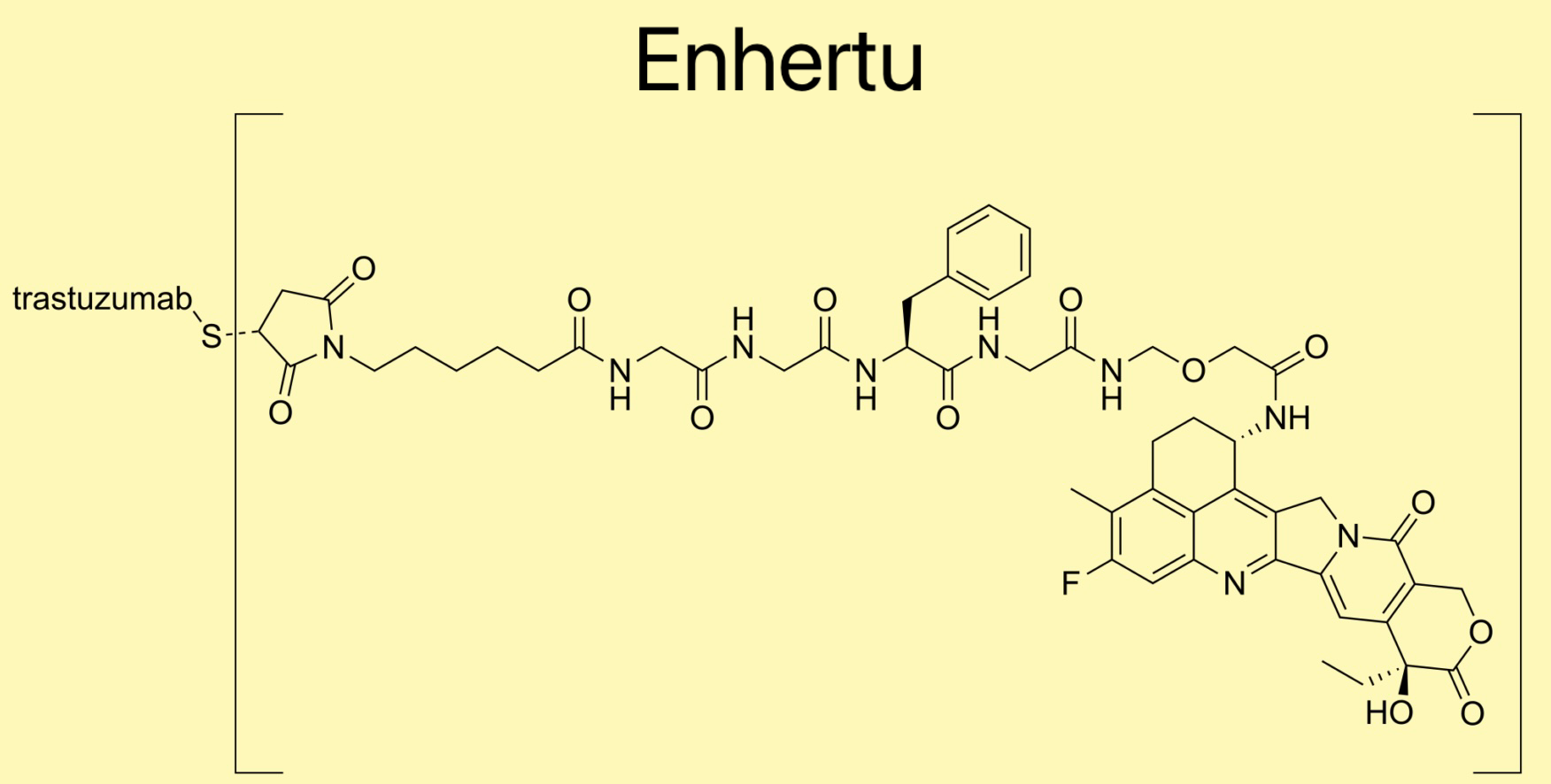
METUPUK are delighted by the Scottish Medicines Consortium (SMC) acceptance of Enhertu (trastuzumab deruxtecan) for routine use on the NHS. The Scottish Medicines Consortium (SMC) has accepted: Trastuzumab deruxtecan (Enhertu)

Hi. My name is Gita and I have been a Therapeutic Radiographer for nearly 30 years. I’d like to share my professional and personal experiences of treating both primary and secondary breast cancer.