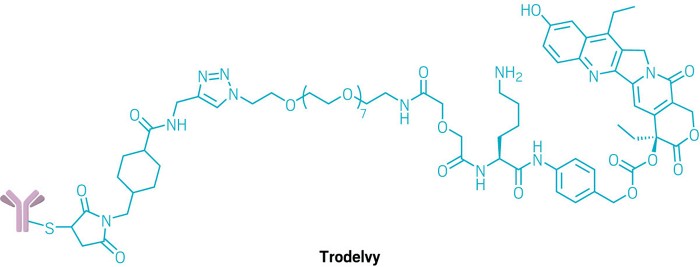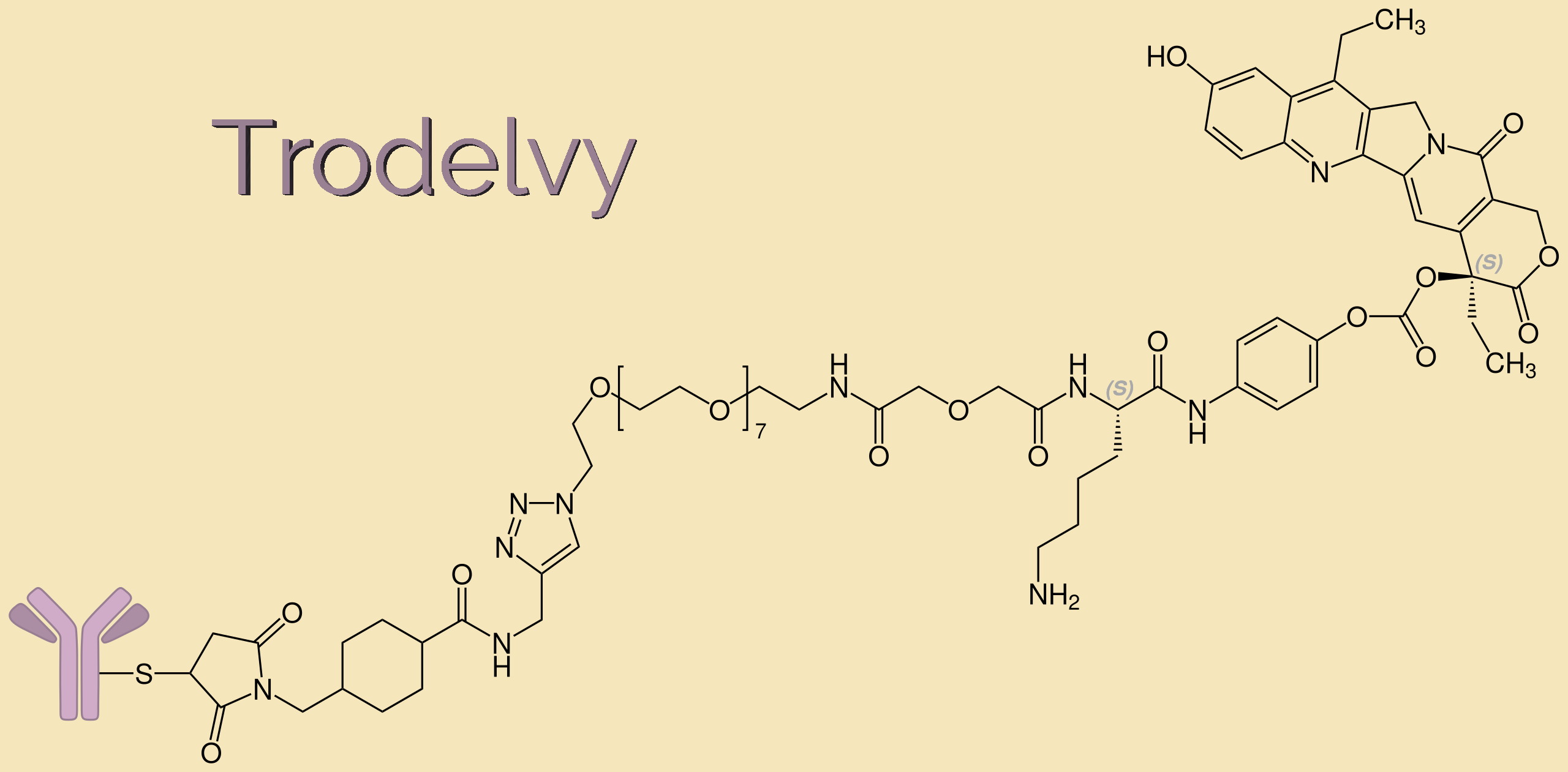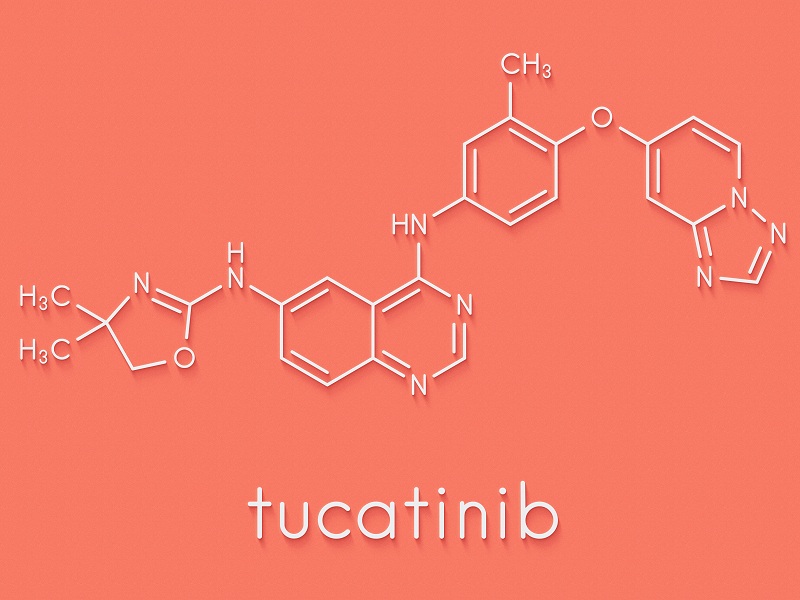
METUPUK are delighted for the approval of Trastuzumab deruxtecan (Enhertu) for treating HER2-positive unresectable or metastatic breast cancer in adults who have received one or more prior anti-HER2 based therapies approved for use through the Cancer Drugs Fund in NHS in England.